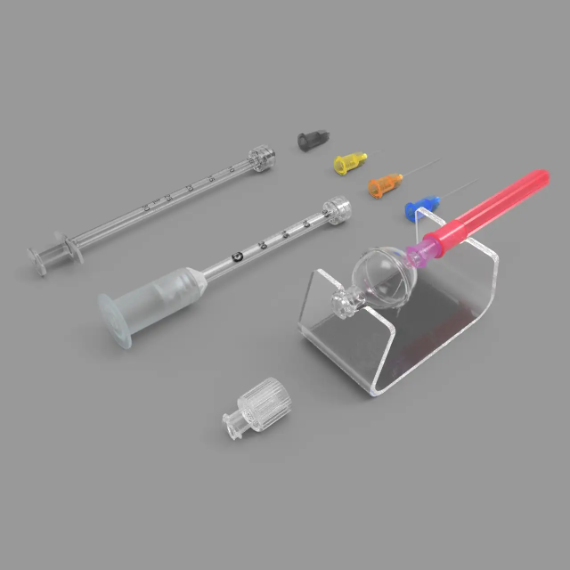
When certain syringe manufacturers sent out field safety notices, stating their syringes are not recommended for intravitreal applications, clients often asked us whether our Zero Residual™ products comply to the intravitreal standard. Unfortunately, to date, no such standard exists. However, as our goal at SJJ Solutions is to provide the highest quality to our customers, we are providing USP <789> certificates with every batch.
What is the USP <789>?
Ophthalmic solutions should be essentially free from particles. As more and more intravitreal injections are taking place globally and papers are being published on some of the complications associated to these injections, there is clinical evidence to suggest that particles are a real problem. Avoiding the chance on injecting particle matter such as silicone oil, foreign particles and protein aggregates should therefore be a top priority. The USP <789> is similar to the European Pharmacopeia Ch. 2.9.19.
USP <789> strictly limits subvisible particles in ophthalmic solutions. Light Obscuration and in some cases Microscopic Membrane are used to identify particles and their respective sizes. The Zero Residual™ products fall well within the given limits, which provides a good baseline for our clients using the products at point-of-care.
Particle limits per milliliter – LO method:
| Diameter | ||
|---|---|---|
| ≥ 10 µm | ≥ 25 µm | |
| Number of particles | 50 per mL | 5 per mL |
Even if an anti-VEGF drug has low particle levels in a vial, if it is compounded by means of a reservoir/transfer syringe and prefilled into the final syringe, it may become contaminated by particulate matter from these syringes used and the injected drug may not meet USP<789> limits.
SJJ Solutions provides the Zero Residual™ Bubble Adapter and Silicone Free 5µm filter needles to allow a full silicone free/low trail, from vial to eye.