Introduction
Intravitreal injections (IVIs) are widely used to deliver therapeutic agents for various retinal conditions. The syringe accuracy is a critical factor in achieving optimal treatment outcomes and minimizing potential risks in IVIs, such as unpredictable variation in drug volume (and thereby therapeutic effect) and intraocular pressure (IOP) rise [1,2,3,4,5]. Smaller volumes than the typical 0.05 mL, albeit harder to deliver with common 1-mL syringes, could mitigate the impact on IOP and drug wastage.
In this study, we evaluated the accuracy and residual volume of three syringe models that can potentially administer very small volumes intravitreally.
Materials and methods
We investigated three syringe models (Fig. 1): BD Plastipak 1 mL luer slip (Becton Dickinson S.A., Madrid, Spain), Omnifix-F 1 mL (B. Braun Medical Inc., Melsungen, Germany), and Zero Residual Enhanced Dosing Technology (EDT) 0.2 mL (SJJ Solutions BV, Den Haag, Netherlands). Notably, the last model is designed to deliver very small volumes accurately. The BD Plastipak and the Omnifix-F were tested with a standard 30-gauge BD PrecisionGlide needle (Becton Dickinson S.A., Curitiba, Brazil), whereas the Zero Residual EDT was assembled with a 30-gauge Zero Residual needle (SJJ Solutions BV, Den Haag, Netherlands).
A trained member of the research team prepared and assessed each syringe-needle setup. Distilled water was used as the injection fluid, and volumes of 10, 20, 25, and 50 µL were tested. The weight of the syringe-needle setup was measured before and after liquid aspiration and after ejection. The extra syringe-needle weight after liquid ejection defined the residual volume. Each simulation utilized a new syringe and needle. The weight was measured with an analytical balance (OHAUS Model PR224, Parsippany, NJ). The study was conducted at a stable 23 °C room temperature and 70% humidity.
Results
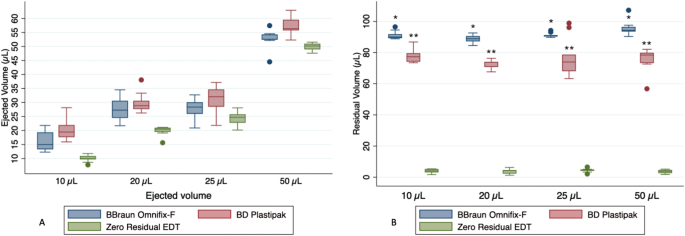
The Zero Residual EDT syringe demonstrated significantly lower volumes delivered and closer to the target volume across all tested volumes compared to the BD Plastipak syringe (p < 0.001), as shown in Fig. 2A. In the comparison between the Zero Residual EDT and the B. Braun Omnifix-F syringe, significant differences were observed at volumes of 10 and 20 µL. Furthermore, a statistically significant difference in residual volume was found between the three syringes for all volumes, with the Zero Residual EDT syringe exhibiting the lowest residual volume (Fig. 2B).
Discussion
The findings of this study highlight the importance of both syringe accuracy and residual volume in the setting of the very small volume delivered during IVIs. The Zero Residual EDT syringe performed better than the BD Plastipak and Omnifix-F syringes with regard to both accuracy and residual volume. The ability to accurately administrate very small volumes is a prerequisite for developing intraocular drugs with lower risk of increased IOP, which, for instance, has been associated with current pre-filled aflibercept syringes [4, 5]. Moreover, minimizing the residual volume of the syringe-needle setup can lead to considerable cost savings by reducing medicine waste. Further research and development in the field of syringe design for IVIs are warranted to explore the full potential of achieving accurate drug delivery, thereby optimizing the benefits and minimizing the risks of this standard ophthalmic procedure.
Data availability
Additional data will be made available upon reasonable request.
References
- Melo GB, Cruz NFSD, Emerson GG, Rezende FA, Meyer CH, Uchiyama S, et al. Critical analysis of techniques and materials used in devices, syringes, and needles used for intravitreal injections. Prog Retin Eye Res. 2021;80:100862.Article CAS PubMed Google Scholar
- Agra LLM, Sverstad A, Chagas TA, Araújo RH, Oliveira LG, Kristianslund O, et al. Accuracy, precision, and residual volume of commonly used syringes for intravitreal injections and the impact on intraocular pressure. Ophthalmol Ret. 2023;S2468-6530:00254–3.Google Scholar
- Meyer CH, Liu Z, Brinkmann C, Rodrigues EB, Helb HM. Accuracy, precision and repeatability in preparing the intravitreal dose with a 1.0-cc syringe. Acta Ophthalmol. 2012;90:e165–166.Article PubMed Google Scholar
- Guest JM, Malbin B, Abrams G, Parendo A, Das S, Okeagu C, et al. Accuracy of intravitreal injection volume for aflibercept pre-filled syringe and BD Luer-Lok one-milliliter syringe. Int J Retin Vitreous. 2022;8:27.Article Google Scholar
- Gallagher K, Raghuram AR, Williams GS, Davies N. Pre-filled aflibercept syringes-variability in expressed fluid volumes and a case series of transient central retinal artery occlusions. Eye. 2021;35:2899–900.Article CAS PubMed Google Scholar
Author information
Authors and Affiliations
- Hospital de Olhos de Sergipe, Aracaju, SE, BrazilGustavo Barreto Melo & Lydianne Lumack do Monte Agra
- Federal University of São Paulo, São Paulo, SP, BrazilGustavo Barreto Melo, Lydianne Lumack do Monte Agra & Carsten Meyer
- Federal University of Sergipe, Lagarto, BrazilLucas Andrade Santos
- Philipps University of Marburg, Marburg, GermanyCarsten Meyer
- Department of Ophthalmology, Oslo University Hospital and University of Oslo, Oslo, NorwayMorten Carstens Moe & Øystein Kalsnes Jørstad
Contributions
Conceived and designed the study: GBM, CM; analysed the data: GBM, CM, ØKJ, MCM; wrote the paper: GBM, critically revised the manuscript: GBM, LAS, LLMA, CM, ØKJ, and MCM; all authors read and approved the final manuscript.
Corresponding author
Correspondence to Gustavo Barreto Melo.
Ethics declarations
Competing interests
GBM has been a consultant to Molecular Partners, Pine Pharmaceuticals, TriboFilm Research, and SJJ Solutions; and has been member of Bayer, Roche and West Pharmaceutical advisory boards. MCM is part of a collaborative agreement with SJJ Solutions for development of the Zero Residual Silicone Oil-free 0.2 mL syringe, has been a member of advisory boards at Bayer, Roche, Novartis, Allergan and Apellis and has received lecture fees from Bayer and Roche. ØKJ has applied patent for a glaucoma stent, is part of a collaborative agreement with SJJ Solutions for development of the Zero Residual Silicone Oil-free 0.2 mL syringe, has been member of Bayer, Roche, and Allergan advisory boards, and has received lecture fees from Bayer and Allergan. The other authors declare there are no competing interests in relation to this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.